Glutamine as a Fuel in Cancer Cells
Glutamine plays a crucial role in cancer cell metabolism and proliferation. Here's an overview of how glutamine is utilized in cancer cells:
Metabolic Fuel and Biosynthesis
Cancer cells exhibit increased consumption of and dependence on glutamine compared to normal cells[1][3]. Glutamine serves as an important metabolic fuel and biosynthetic precursor in cancer cells through several key pathways:
TCA Cycle Anaplerosis
Glutamine is converted to α-ketoglutarate, which enters the tricarboxylic acid (TCA) cycle to replenish intermediates and support energy production[3][4]. This process, called anaplerosis, is vital for rapidly proliferating cancer cells.
Amino Acid and Protein Synthesis
Glutamine provides nitrogen for the synthesis of other amino acids and nucleotides needed for protein and DNA/RNA production[3][5].
Lipid Biosynthesis
Glutamine-derived citrate can be used for fatty acid synthesis, especially under hypoxic conditions[4].
Redox Balance
Glutamine metabolism contributes to cellular redox homeostasis in cancer cells:
It supports the production of NADPH, which is important for managing oxidative stress[4].
Glutamine is used to synthesize glutathione, a key antioxidant[2].
Signaling Functions
Beyond its metabolic roles, glutamine also influences important signaling pathways in cancer cells:
It activates mTOR signaling, which promotes cell growth and proliferation[6].
Glutamine metabolism suppresses endoplasmic reticulum stress[6].
Metabolic Flexibility
Cancer cells show metabolic flexibility in glutamine utilization:
They can use either glutamate dehydrogenase or aminotransferases to metabolize glutamine-derived glutamate, with aminotransferases potentially supporting a more biosynthetic phenotype[6].
Some cancer cells can synthesize glutamine de novo when extracellular glutamine is limited[1].
Therapeutic Implications
The dependence of many cancer cells on glutamine, often termed "glutamine addiction," has made glutamine metabolism an attractive target for cancer therapy[1][3]. Strategies include:
Inhibiting glutamine transporters like SLC1A5 to reduce glutamine uptake[5].
Targeting glutaminase (GLS), the enzyme that converts glutamine to glutamate[3][4].
Exploiting the metabolic vulnerabilities created by glutamine dependence in combination therapies[6].
Understanding glutamine metabolism in cancer cells has opened up new avenues for therapeutic intervention, although the complexity and adaptability of cancer metabolism present ongoing challenges for developing effective treatments.
Citations:
[1] https://www.healthline.com/health/glutamine-and-cancer
[2] https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2024.1345522/full
[3] https://www.nature.com/articles/s12276-023-00971-9
[4] https://pmc.ncbi.nlm.nih.gov/articles/PMC5746034/
[5] https://jeccr.biomedcentral.com/articles/10.1186/s13046-024-02994-0
“DON” Therapy
6-Diazo-5-oxo-L-norleucine (DON) is a glutamine antagonist that has been studied extensively as a potential anticancer agent. Here are the key points about DON:
Chemical Properties and Mechanism
DON is an amino acid analog of glutamine with a diazo group[1][5].
It acts as a broad inhibitor of glutamine-utilizing enzymes by mimicking glutamine[1][9].
DON irreversibly binds to and inhibits enzymes like glutaminase, amidotransferases, and others involved in glutamine metabolism[9].
Effects on Cancer Cells
DON broadly blocks glutamine-dependent reactions critical for cancer cell growth, including nucleic acid synthesis, protein synthesis, and energy metabolism[1].
It inhibits multiple enzymes in purine and pyrimidine synthesis pathways[4].
DON has shown anticancer efficacy in preclinical models against various tumor types[3][5].
Clinical History
DON was first isolated from Streptomyces bacteria in the 1950s and tested clinically for cancer treatment[5][9].
Early clinical trials in the 1950s-60s using low daily doses showed some antitumor activity[3].
Later trials using higher intermittent doses were limited by toxicity, particularly gastrointestinal side effects[3][4].
Clinical development was halted due to dose-limiting toxicities and lack of tumor selectivity[1][3].
Recent Developments
There is renewed interest in DON due to the recognition of glutamine dependence in many cancers[4].
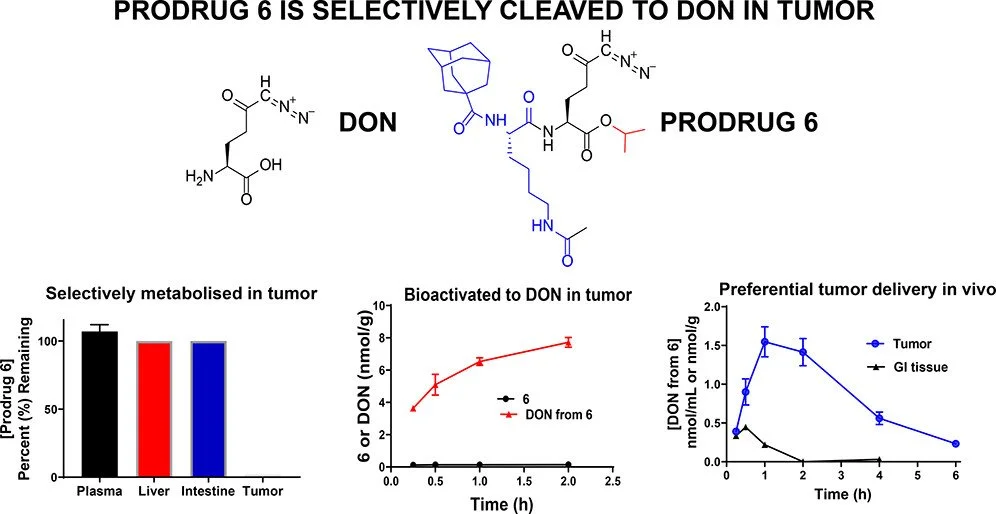
Prodrug forms of DON (like JHU083) have been developed to improve tumor selectivity and reduce toxicity[1][3].
These prodrugs are designed to be activated preferentially within tumors[1][7].
Recent studies show DON and its prodrugs can have immunomodulatory effects in the tumor microenvironment[11].
Challenges and Potential
DON has a narrow therapeutic window due to on-target effects in normal glutamine-utilizing tissues[9].
Combining DON or its prodrugs with other therapies (like immunotherapy) is being explored to enhance efficacy[8][11].
Improved bioanalytical methods have been developed to better quantify DON in tissues for pharmacokinetic studies[9].
In summary, DON is a potent glutamine antagonist with proven anticancer effects, but its clinical use has been limited by toxicity. New prodrug formulations and combination strategies are being investigated to harness its therapeutic potential while minimizing side effects.
Citations:
[1] https://pmc.ncbi.nlm.nih.gov/articles/PMC8025739/
[2] https://link.springer.com/article/10.1007/s12274-024-6534-4
[4] https://aacrjournals.org/mct/article/17/9/1824/92489/We-re-Not-DON-Yet-Optimal-Dosing-and-Prodrug
[5] https://en.wikipedia.org/wiki/6-Diazo-5-oxo-L-norleucine
[6] https://drugdiscovery.jhu.edu/our-projects/glutamine-antagonist/
Glutamine Inhibition
Several glutamine blockers have shown promise in preclinical studies and clinical trials. Here are some of the top glutamine blockers used in targeting cancer cell metabolism:
Glutaminase Inhibitors
Glutaminase (GLS) is a key enzyme that converts glutamine to glutamate, which is then used in various metabolic pathways. Inhibiting GLS has emerged as a major strategy for blocking glutamine metabolism in cancer cells.
1. CB-839 (Telaglenastat): This is one of the most advanced glutaminase inhibitors in clinical development[1][2]. CB-839 is a potent, selective, and orally bioavailable inhibitor of GLS1. It has shown promising results in preclinical studies and is currently being evaluated in multiple clinical trials for various cancer types, including breast cancer, renal cell carcinoma, and hematological malignancies.
2. BPTES (Bis-2-(5-phenylacetamido-1,3,4-thiadiazol-2-yl)ethyl sulfide): This was one of the first GLS inhibitors developed[6]. While less potent than CB-839, BPTES has been widely used in preclinical studies to demonstrate the potential of glutaminase inhibition in cancer treatment.
3. Compound 968: Another GLS inhibitor that has shown efficacy in preclinical models[6]. It works as an allosteric inhibitor of GLS1.
Glutamine Transporter Inhibitors
Blocking glutamine uptake by targeting its transporters is another strategy to disrupt glutamine metabolism in cancer cells.
4. V-9302: This compound targets ASCT2 (SLC1A5), a major glutamine transporter overexpressed in many cancers[1][5]. V-9302 has shown promise in preclinical studies, particularly in combination with other metabolic inhibitors.
Prodrugs of Glutamine Analogs
5. JHU083: This is a prodrug of 6-diazo-5-oxo-L-norleucine (DON), a glutamine analog[3]. JHU083 was designed to improve the delivery and reduce toxicity of DON. It has shown impressive results in preclinical studies, including the ability to enhance anti-tumor immune responses.
Combination Strategies
Many studies have found that combining glutamine blockers with other therapies can enhance their efficacy:
CB-839 has been tested in combination with various drugs, including PARP inhibitors, mTOR inhibitors, and immune checkpoint inhibitors[2][7].
Combining glutaminase inhibitors with drugs targeting other metabolic pathways, such as glycolysis inhibitors, has shown synergistic effects in some cancer models[4].
Emerging Approaches
Research is ongoing to develop new and improved glutamine blockers:
Dual inhibitors targeting both GLS1 and GLS2 isoforms are being explored[6].
Strategies to overcome resistance mechanisms to glutamine blockade are an active area of investigation[5].
It's important to note that while these glutamine blockers have shown promise, their efficacy can vary depending on the cancer type and genetic background. Ongoing clinical trials will provide more insights into their effectiveness as cancer treatments.
Citations:
[1] https://elifesciences.org/articles/56749
[4] https://pmc.ncbi.nlm.nih.gov/articles/PMC5746034/
[5] https://www.nature.com/articles/s12276-023-00971-9
[6] https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2024.1345522/full
[7] https://www.sciencedirect.com/science/article/pii/S1367593121000065
Yamashita AS, Rosa M da C, Stumpo V, et al. The glutamine antagonist prodrug JHU-083 slows malignant glioma growth and disrupts mTOR signaling. Neuro-Oncol. Adv. 2020;3(1):vdaa149.
Epigallocatechin Gallate (EGCG)
Epigallocatechin gallate (EGCG) has been shown to affect glutamine metabolism in cancer cells. Here are some key points on how EGCG impacts glutamine metabolism in cancer:
1. EGCG inhibits glutamate dehydrogenase (GLUD1/GDH):
EGCG acts as an inhibitor of GLUD1, which catalyzes the conversion of glutamate to α-ketoglutarate in the glutaminolysis pathway.
By inhibiting GLUD1, EGCG can suppress the proliferation of various cancer cell types, including neuroblastoma, glioma, and colorectal cancer cells.
2. Suppression of metabolic reprogramming:
EGCG has been found to suppress multiple metabolic reprogramming pathways in cancer cells, including glutamine metabolism.
It can inhibit glutamine uptake and utilization by cancer cells.
3. Targeting glutaminase:
EGCG's ability to suppress glutamine metabolism suggests it may also impact glutaminase activity, which is the first enzyme in the glutaminolysis pathway.
4. Affecting redox homeostasis:
Glutamine metabolism plays a crucial role in maintaining redox balance in cancer cells. By interfering with glutamine metabolism, EGCG may disrupt the redox homeostasis of cancer cells.
5. Potential synergistic effects:
Given EGCG's ability to modulate glutamine metabolism, it may have potential synergistic effects when combined with other glutamine metabolism inhibitors or cancer therapies.
6. Impact on tumor microenvironment:
By affecting glutamine metabolism, EGCG may also influence the tumor microenvironment, as glutamine plays important roles in immune cell function and the metabolic interplay between cancer cells and surrounding tissues.
Citations:
[1] https://pmc.ncbi.nlm.nih.gov/articles/PMC7226503/
[2] https://www.frontiersin.org/journals/pharmacology/articles/10.3389/fphar.2024.1345522/full
[3] https://www.frontiersin.org/journals/immunology/articles/10.3389/fimmu.2024.1331641/full
[4] https://www.nature.com/articles/s12276-023-00971-9
[5] https://link.springer.com/chapter/10.1007/978-3-030-65768-0_2